3d transition metal oxides for use as electrode materials
Project description:
a) Fe2(MoO4)3 has a theoretical capacity 2.5 times higher than that of graphite. The open anti-Nasicon structure of Fe2(MoO4)3 is well suited for the incorporation of 2 lithium ions per formula unit. Morphology design and the introduction of conductive carbon structures can increase the capacitance of the electrode even more. Polyanion compounds such as Fe2(MoO4)3 can also deliver high voltages, which is due to a polarization of the Fe-O bond, which lowers the redox potential of Fe2+ / Fe3+. The major volume changes associated with the incorporation of lithium are problematic concerning the use as electrodes in lithium-ion batteries. The intercalation takes place in two stages via LiFe2(MoO4)3 and involves a phase change from monoclinic to orthorhombic. If the lithium is replaced by sodium, the intercalation mechanism is different due to the different size, and the conversion takes place in a single phase, similar to solid solution formation with slight structural changes, since the sodium ion fits into the spaces between the polyhedra of the Fe2(MoO4)3 -structure. In addition to usage as anode material, the intercalation compounds with lithium / sodium are also promising as cathode material instead of LiCoO2 / NaxCoO2. Nanostructured Fe2(MoO4)3 is to be investigated as anode and cathode material in lithium and sodium ion batteries. For this purpose, samples from different syntheses with different morphology are to be tested in order to figure out the influence of the particle morphology.
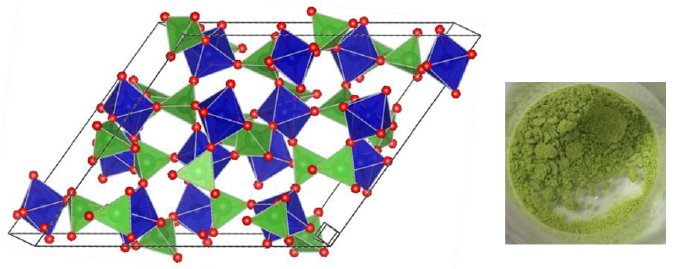
Crystal structure of Fe2(MoO4)3 and photo of the powder
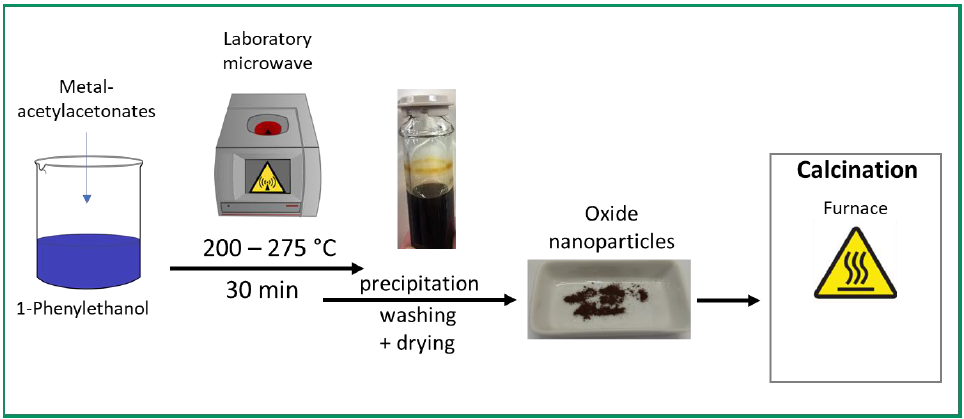
b) In addition to the problem of limited deposits of cobalt and lithium, which are required for lithium ion accumulators, another major problem for the use of batteries in EVs is their high weight compared and at the same time relatively short range. Therefore, the use of batteries with a high energy density is advantageous in order to reduce the weight. Therefore, metal-air batteries are of particular interest, since a half-reaction involves the conversion of atmospheric oxygen on a porous electrode. The use of iron-air batteries, which have an open-circuit potential of 1.28 V, is particularly interesting. Iron is both cheap and available in large quantities. However, high requirements are placed on the positive electrode for the oxygen side. High potentials are necessary for the development of oxygen, below which the electrode has to be electrochemically stable. On the other hand, it should be able to catalyze both the development and the reduction of oxygen with the lowest possible overpotentials. There is still a great need for the development of such new catalysts, since the previously used ones usally contain noble metals or they are not able to catalyze both reactions efficiently. Spinels (often in combination with carbon) can be used as transition metal catalysts, with NiCo2O4 being particularly promising. Other cobalt-containing materials, such as Co3O4, have also shown some high stabilities and low overpotentials. The good catalytic properties of some spinels, such as CuCo2O4 in the development and reduction of oxygen, are promising for use as electrodes in metal-air batteries. Therefore, new low-cobalt spinel nanoparticles are to be produced as catalysts for use in air batteries using anhydrous microwave synthesis, which is already established in the Marschall Group. The effect of different synthesis parameters is to be investigated. The composition of the resulting spinels should be varied systematically in order to obtain mixed oxide spinels of several metals in order to control the efficiency in the air electrodes.
The project is closely affiliated with the Roth-Papastavrou tandem.
Project duration: 3 years
Principal investigator:
- Prof. Dr. Roland Marschall, Chair of Physical Chemistry III
PhD student:
- Judith Zander, M. Sc.